
Copyright © 2026, Molsoft LLC
Feb 18 2026
Google Search:
Keyword Search:| Prev | ICM Language Reference Sequence, searches and alignments | Next |
[ Search prosite | Binding site analysis | Pdb sequence generation | Pdb merge | Search pdb headers ]
How to search all Prosite patterns in your sequence |
Use macro searchSeqProsite. For example:
read pdb "2dhf" make sequence a_1.1 # sequence of a PDB structure show sequence find prosite 2dhf_a # 2dhf_a is the sequence of the proteinSee also find prosite, find pattern and read prosite.
How to identify binding pockets |
There are three algorithms (A, B, and C) with ICM which can identify pockets:
| option | target | macro |
|---|---|---|
| A | closed pockets | `icmCavityFinder |
| B | almost closed pockets | --{make map potential} , etc., see below |
| C | pockets with good ligand-binding potential | `icmPocketFinder |
Example:
read pdb "1a28" delete a_!1,2 convert delete a_2 icmPocketFinder a_ 3. yes no |
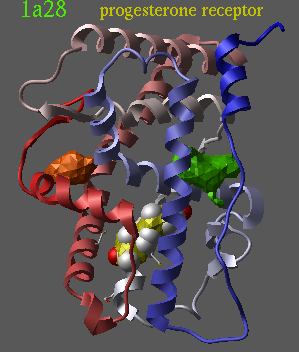
|
In the following example we find an almost closed pocket which can not be identified with icmCavityFinder .
read pdb "1fm6" # read the 'a' chain of RXR delete a_!1,9 # keep the RXR and its ligand only make map potential a_1 Box( a_ 1. ) 1. # grid size 1.5 A make grob m_atoms exact 0.1 solid split g_atoms cool a_ display g_atoms2 reverseIf you have problems with identifying pockets, change the grid size, the threshold level for make grop m_atoms , or try to convert object to the ICM type (the conversion will add hydrogens and make the object more dense).
How to generate a non-redundant list of PDB sequences |
The following script is a skeleton of the provided script _mkUniqPdbSeqs which is somewhat more automated.
l_commands=no
errorAction="none" # if something goes wrong do not
# interrupt the loop
s_pdbDir = "/data/pdb/" # make sure you have correct path
pdbDirStyle = 4 #
read sarray s_pdbDir+"/derived_data/index/source.idx"
# you need a list of all pdb-entries
# (4 char. code per line will do)
source = Tolower(Trim(Field(source,1)))
n=Nof(source)
for i=5,n
read pdb sequence resolution source[i]
# append resolution to the chain name (like 9lyz_a19)
endfor
group sequence "*" uniqSeqs unique 0.1 delete
# cutoff inter-sequence
# distance 0.1 (dissimilar by more than 10%)
#
# Other possibilities
#
# group sequence uniqSeqs unique 5 # if two seqs differ by more
# # than 5 mutations
# group sequence uniqSeqs unique # throw away only identical
# # sequences
#
write sequence s_inxDir + "/pdb1.seq"
# actual sequences for searches
write Name(uniqSeqs) "chainList"
# list of protein chains if you need it
quit
How to merge several pdb files |
The simplest way to merge two pdb files is to read them as separate objects and the use the move a_1. a_2. command. Example:
read pdb "1crn" read pdb "1d48" move a_2. a_1. # merges objects write pdb a_1. "both" # saves both files in pdb format write object a_1. # saves merged object in compact binary form
Before or after merging, the objects can also be edited, translated to a new position, rename chains, change residue numbers etc. Example:
read pdb "1d48" delete a_w* delete a_2 # delete the second chain read pdb "1crn" delete a_/33:99 # delete a C-term. part of crambin move a_1. a_2. # merge the remains write object a_
If you want to re-engineer a polypeptide chain of a protein, using two pdb-files, e.g. to transplant one part of a protein to another and restore the bonding connectivity, you may use the modify command:
read pdb "1crn" # one pdb read pdb "1cbn" # similar protein modify a_1./20:25 a_2./20:25 # grafts a loop from 2nd object to the 1st one write pdb a_1. "combo"
How to search headers of the PDB entries |
There is an PDB.tab file which contains one line header descriptions of all the entries. Now you have three ways of doing it:
- In unix: grep -i kinase PDB.tab
- From ICM: you have more possibilities:
read table s_icmhome+"data/inx/PDB.tab" # or in s_userDir+"inx/" show PDB.head ~ "kinase" # or show PDB.head ~ "*kinase*" # or show PDB.comp ~ "kinase*" # regular expressions # You can also a=PDB.head~"kinase" for i=1,Nof(a) nice PDB.ID pause delete a_*. endfor
- Use the gui (Find.In PDB.By Keyword..)
| Prev Cavity analysis | Home Up | Next Molcart |